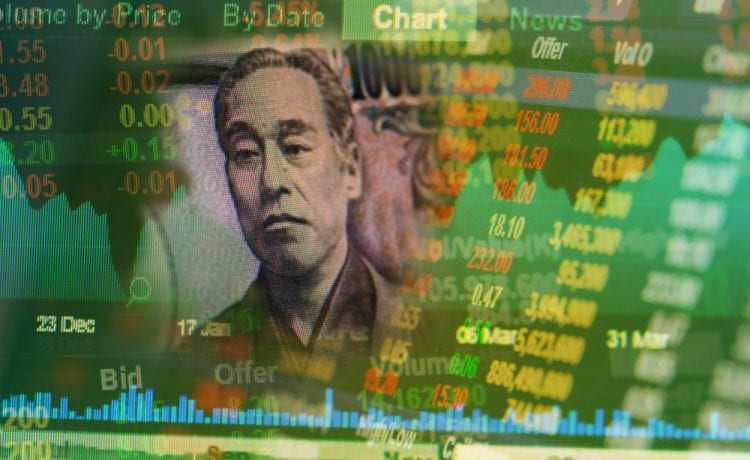
Nikkei share average was flat at 28,863.88, while the broader Topix dropped 0.14% to 1,946.45
Shares in Japan were flat on Thursday, as traders focussed on the latest statements from U.S. Fed, while investors were on the sidelines amid uncertainty over domestic corporate earnings.
Nikkei share average was flat at 28,863.88 at 0015 GMT, while the broader Topix dropped 0.14% to 1,946.45.
Although the market has been recovering from last week’s losses, triggered by a hawkish shift in the U.S. Fed’s policy guidance, it is starting to lose traction, analysts said.
U.S. inflation could stay at high levels, so we will need more data on inflation, wages and employment. And, quarterly earnings will be a few weeks away, the market is running out of trading factors, Nobuhiko Kuramochi, senior strategist at Mizuho Securities said.
Two Fed officials said a period of high inflation in the United States could last longer than anticipated, a day after Fed Chair Jerome Powell played down rising price pressures.
Many hospitality-related shares, which led index’s gains before the Fed meeting outcome, have now succumbed to profit-taking.
West Japan Railway shed 1.9% while department store operators Isetan Mitsukoshi and Takashimaya declined 1.9% each.
Sumitomo Forestry lost 2.3% after the company announced a plan to sell new 16 million shares, which amount to 8.7% of its existing shares to raise up to 37.1 billion yen ($334.32 million).
Toshiba erased early losses to trade 0.4% higher after financial magazine Diamond reported Kioxia Holdings Corp plans to list as early as in September. Toshiba holds a 40.6% stake in Kioxia, formerly known as Toshiba Memory.
Some growth shares outperformed after U.S. tech stocks hit a record high overnight.
Electronic parts maker Sumco gained 2.4% and Minebea Mitsumi advanced 1.8%.
Mercari gained over 10% after the flea market app operator forecast a net annual profit of 5 billion yen ($0.045 billion), its first annual profit since its listing in June 2018.
Eisai advanced 3.7% after the drugmaker said U.S. regulator had granted breakthrough therapy designation to their experimental therapy, lecanemab, for patients with early Alzheimer’s.